Rejected Specimen Policy
Collection Procedure:
LTD: Rejected Specimen Policy Version 7
Purpose:
To provide guidelines for specimen labeling and specimen rejection to the
laboratory and all Akron Children’s Enterprise.
Clinical Significance:
Wrong-patient errors can lead to significant medical error and patient harm.
Patient identification errors can occur in all stages of diagnosis and treatment,
especially at the time of specimen collection. Mislabeled specimens create the
greatest risk for misdiagnosis and for this reason will routinely NOT be
processed. Irretrievable specimens are the only exception to this rejection policy.
Due to the inherit risk to the patient,
ABSOLUTELY NO mislabeled blood bank samples will be accepted by our
laboratory. Mislabeled Blood Bank samples cannot be used regardless if
considered irretrievable. Specimens with only one complete patient
identifier may NOT be accepted regardless if the collector is willing to
come to the laboratory and provide and label the specimen with a second
identifier
Policy:
Positive patient identification must occur throughout the collection, testing and
reporting process for all specimens submitted to the laboratory.
The use of two (2) positive patient identifiers must be used throughout the
entire collection and testing process. Acceptable patient identifiers are:
- Name
- Date of birth
- Medical record number
All specimens must be labeled in the presence of the patient, immediately
after collection and within site of the patient. Confirmation of the patient
identifiers should occur at this time.
The physical label should be placed on the portion of the container that
contains the specimen. DO NOT label a removable portion of the container.
Specimens may be rejected that do not meet patient preparation, collection,
labeling, processing, storage or transport criteria. Specific criteria are
available in the Laboratory Test Directory.
All rejected specimens will be communicated to the physician/nursing unit.
Documentation will be entered into the LIS.
Proper Labeling Requirements:
ALL specimens, regardless of type, should be labeled immediately after
collection before leaving the patient’s room/bedside.
ALL labels MUST include:
- Two (2) patient identifiers
- Collection date
- Collector identification
If lab generated collection labels are not used then the specimen MUST be
accompanied by an audit trail or requisition that contains the same two (2)
patient identifiers used to label the specimen.
ALL BLOOD BANK SPECIMENS are required to be labeled correctly and
MUST be accompanied by a double-signed blood bank requisition.
- Full instructions are available for Blood Bank specimen acceptability in
“Blood Bank Guidelines and Procedures.” There is a separate policy for
Akron and Mahoning Valley Campuses. Links are provided under
“Additional Procedures” in this policy.
Definitions:
Collector – trained caregiver who is present with the patient during collection
of the specimen. Each collector should use their employee number or other
identifier that is traceable to them when collecting specimens.
Mislabeled specimen – any specimen with incorrect patient information. The
incorrect information could be one (1) or both patient identifiers duplicate
labeling (labeling with two (2) or more patient’s information) or missing label.
- ANY specimen for Blood Bank WILL NOT be accepted if mislabeled.
- Discrepant information between the request form and specimen can be
considered mislabeled when the labeled specimen cannot be verified.
Suboptimal specimen – specimens that do not meet the ideal requirements of
collection. Some suboptimal specimens can be used for testing, for example
a sample with one patient identifier that the collector is willing to complete the
labeling. Other suboptimal specimens cannot be used for testing, such as a
clotted sample. Some interpretation may be needed on suboptimal
specimens. Contact the supervisor with any questions. The following list is
meant as examples and is not all inclusive as each test has its own
requirements:
- Improperly collected and/or preserved samples (i.e. clotted, hemolyzed,
contaminated)
- Volume not sufficient for testing
- Specimen container contaminated by specimen (leaking specimen)
- Patient not properly prepared for testing (i.e. not fasting)
- Specimen labeling issues
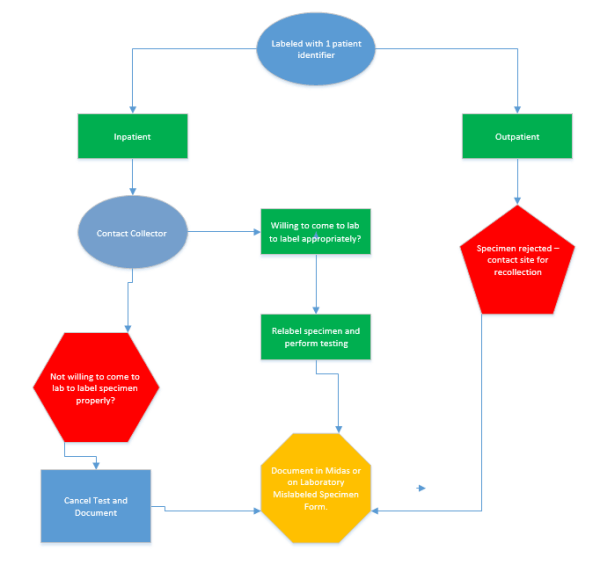
Specimens with only one patient complete patient identifier may be
accepted ONLY if the specimen collector is willing to come to the
laboratory and provide and label the specimen with a second identifier
Specimens received without collection times if necessary (i.e. glucose
tolerance)
Specimens without collector’s identification
Patient Identifier – a unique piece of information that can identify a patient.
Acceptable patient identifiers are name, date of birth, medical record number,
blood bank identification bands (if used). A room or bed number is not an
acceptable patient identifier.
Specimen – any sample used for medical testing. Specimens are also
commonly referred to as samples.
Label – a preprinted or handwritten material attached to a specimen
identifying who the sample was taken from. At Akron Children's we
have generated labels that will supply the patient and test information
required. If generated labels are not available all samples may be labeled
with legible handwriting. Two identifiers are still required.
Laboratory Test Directory (LTD) – a listing of offered tests available on the
myKidsnet homepage under Departments and Laboratory
Rejected Specimen – Specimen that is not acceptable for testing in the
laboratory. Rejected specimens may be due to labeling error, quality of
sample of quantity of sample.
Irretrievable Specimen – specimens that are not reproducible due to clinical
state and are critical to the diagnosis or treatment of the patient or specimens
that are difficult or traumatic to obtain. These specimens are the only
exceptions to this policy.
LIS – Laboratory Information System
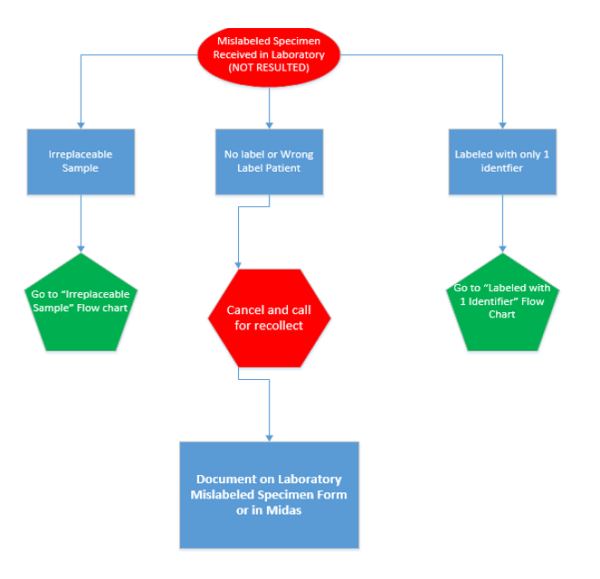
Handling Mislabeled and Unusable Specimens:
1. Upon receipt of a mislabeled or unusable specimen in the laboratory, notify
the physician or nursing unit of the issue with the specimen.
a. If the test cannot be reported, the tests must be canceled in the LIS.
b. The following information MUST be recorded in the LIS:
Collector ID
Who you reported the specimen to
Time you reported unusable specimen
Reason for recollection (mislabeled, clotted, etc.)
c. The test will need to be reordered and recollected at the decision of the
registered provider.
d. If notified that the specimen is irretrievable, see below “Handling of
Irretrievable Specimens”
2. Mislabeled specimens WILL NOT be accepted unless irretrievable. Enter the
mislabeled specimen in Midas and complete a “Mislabeled Specimen
Exception Request Form” (instructions for both can be found as an
attachment to this procedure).
3. Unusable samples include but are not limited to:
a. Clotted samples for whole blood or coagulation testing
b. Hemolysis of samples that can erroneously affect test results
c. Contamination of any sample
d. Specimen quantity not sufficient (QNS) for testing
e. Specimen container contaminated by specimen (leaking container)
f. Wrong anticoagulant/tube type
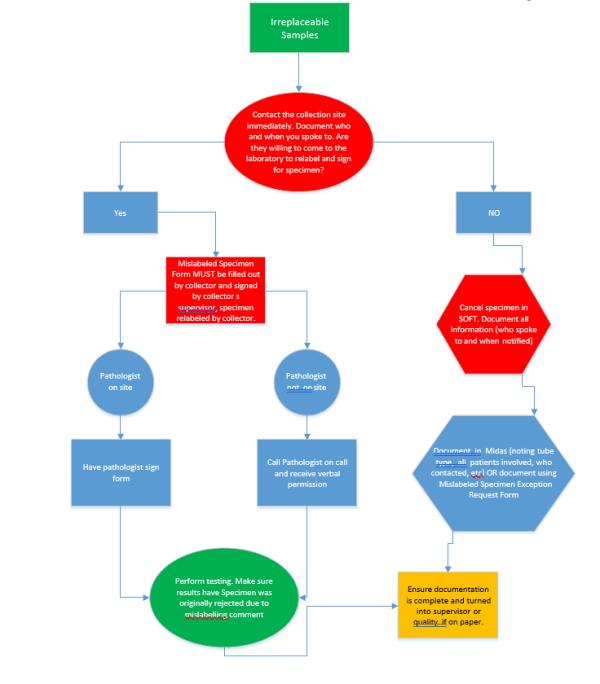
Handling of Irretrievable Specimens:
1. The laboratory defines an irretrievable specimen as one of the following two
(2) types:
- Specimens in which the clinical state cannot be reproduced and the
results are critical to the diagnosis and treatment of the patient.
Example: blood culture obtained prior to an antibiotic start
- Specimens that are traumatic or difficult to obtain. Examples include but
are not limited to:
CSF collection
Bone marrow
Surgical Specimen
Catheter tips
2. Mislabeled specimens will ONLY be processed if they meet the above criteria.
3. For samples collected on campus, the collector MUST come to the laboratory
to label the specimens and complete the “Mislabeled Specimen Exception
Form.” As part of the form completion, documentation on the reverse side of
the form explaining how the collector is certain of specimen identification must
be recorded.
4. For samples collected at outside locations (ex. ACHP’s, Urgent Cares, etc.), a
laboratory employee must have a phone conversation with the collector. If
convinced, the entire specimen package must be sent back to the site for
labeling by the collector.
5. If laboratory personnel are not satisfied by the evidence provided by the
specimen collector and the clinician insists on relabeling, the laboratory will
reach out to a pathologists/medical director to intercede in the situation.
6. ALL reports of testing should include the following statement: “Specimen was
originally rejected due to mislabeling”
7. Mislabeled specimens accepted as irretrievable MUST have a “Mislabeled
Specimen Exception Request Form” filled out and submitted to the laboratory
supervisor, a Midas event on file or both.
Handling Suboptimal Specimens:
1. Suboptimal specimens require some judgment on the part of the performing
laboratorian to determine usefulness of analysis. It is preferable to obtain a
new acceptable specimen. Any question on acceptability should be taken to
the laboratory supervisor.
2. A comment should accompany any results noting any suboptimal specimen
characteristics.
3. Suboptimal specimens could include but are not limited to:
a. A sample with a single positive identifier or lacking collection time or
initials
If the collector is still available on site and willing to properly complete
identification of the sample they may come to the laboratory and
complete the appropriate labeling and the laboratory will accept the
sample.
b. Slightly hemolyzed samples that will not severely affect testing results.
c. QNS specimen that have multiple tests ordered. Contact the care
provider to obtain instructions for which tests to perform first. All
remaining tests unable to perform should be canceled and recollected.
d. Specimens in which the patient was not properly prepared (i.e. fasting).
The laboratory should notify the practitioner to obtain instruction on
whether or not to proceed with testing. If it is determined to not test,
cancel the test making appropriate notations.
Handling Mislabeled Specimens Found After Resulting:
1. Current results may not match previous results. It is important to determine if
the current or previous results are discrepant.
2. Contact the care provider and explain the discrepant results.
3. Discrepant results should be made “Not Available” as soon as possible in the
LIS.
4. A credit should be submitted for testing that is discrepant.
Procedure Notes:
Steps to Complete Laboratory Mislabel Specimen Form
1 Obtain form
2 Notify the collection area of mislabeled specimen
3. Receive the sample in the LIS – Complete and SAVE the specimen collector, Collection date and time and receipt date and time
4. Document on the form and in the LIS who was notified in Step 2 include date and time
5. Follow-up (on form):
Check if the test was canceled (floor must reorder) – When cancelling
an order use the canned message “Mislabel form on file” and record
initials and date of person notified. Any free text comments should be
made on form.
Check if Processing Exception requested (were results reported and
needed to be made “not available”) if so once results were retracted,
print and attach. Complete a no charge form.
6. Submit completed form to Laboratory Supervisor or Manager
7. A Midas event will need to be completed for each mislabeled specimen.
Document the Midas number on the form if it is completed.
Midas Tips
Step Instructions
1. To place a Midas event – go to MyKidsNet at the bottom of the page
under “Quick Links” select “Safety Event Reporting”
2 Select “Test/Procedure/Treatment” from Patient Safety Event
3. You must have patient information to attach your Midas Event to. Select
the date of the event. Using Patient Name or Medical Record Number to select patient
4. Select correct account and OK, then Yes
5. Complete each line with a red asterisk (*). Any line with three (3) dots
(…) has a drop down if you click on the box with the 3 dots. Use these drop downs whenever possible
6. Please be as specific as possible while including as much concise
information as available. Ex: “lab collected specimen labeled with two
different patient identifiers (patient A and patient B)” versus “bad
specimen.” Use professional language; include test names and who notified.
References:
College of American Pathologists, Laboratory General Checklist, Specimen
Collection, Handling and Reporting (pages 19-34). 08/22/2019.
CLSI. Accuracy in Patient and Sample Identification; Approved Guideline. CLSI
document GP33-A. Wayne, PA: Clinical and Laboratory Standards Institute;
2010.